Polish stem cells bank
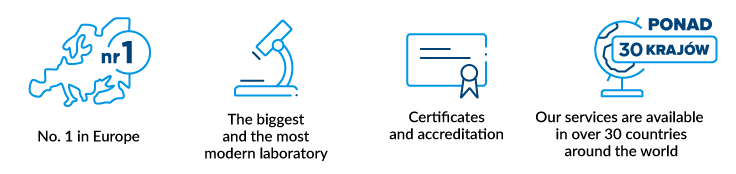
The Polish Stem Cells Bank (PBKM) is the largest stem cells bank in Europe. We store biological material samples from customers in dozens of European countries.
What makes us diffrent?
We bank 4 types of tissues:
When deciding to harvest umbilical cord blood, Parents choose a partner for many years. Therefore, when making a choice, you must be sure that you’re choosing a bank that will perform the best service. The material must remain safe for years, and the whole process must be performed in a manner ensuring that, if necessary, the best transplant clinics in Poland and in the world accept the material for transplantation.
The bank's safety is proven by its scale of operations, technological facilities, qualified staff, certification of quality, experience in storage and preparation of material for transplantation, as well as the trust of doctors and clients.
Mission and vision
- The primary focus of our activities is the collection and storage of cord blood stem cells. These cells can then be used in case of emergency for the treatment of your child, or if genetically compatible, another family member. Our knowledge, as well as our innovative technology for stem cell storage and practical experience as evidenced by tens of thousands of frozen stem cell units, allows us to assure families in case of serious disease.